ATHENA HPV trial
https://www.hpv16and18.com/hcp/athena-hpv-clinical-trial/asc-us-population-results.html
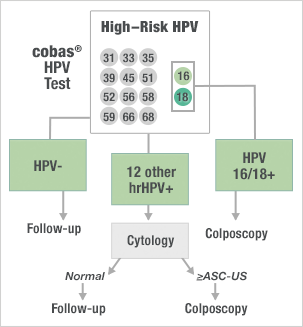
Clinical implications for the cobas® HPV Test from ATHENA in the ASC-US population
- Comparable performance to the prior standard of testing in pooled hrHPV testing
- Significantly improves upon other commercially available HPV tests by individually identifying HPV 16 and 18 while simultaneously detecting 12 other hrHPV genotypes as a pooled result
- The absolute risk of ≥CIN2 was 31.5% among women who were HPV 16 positive
- Pooled hrHPV negative women were at low risk of ≥CIN2
- Women who were HPV 16 positive were more than twice as likely to have ≥CIN2 than those women who were pooled hrHPV positive